41+ Copper And Silver Nitrate Net Ionic Equation US
41+ Copper And Silver Nitrate Net Ionic Equation US. Copper(ii) chloride and sodium hydroxide. Write the complete ionic and net ionic equation for this reaction in aqueous solution in aqueous solution, it is only a few percent ionized.
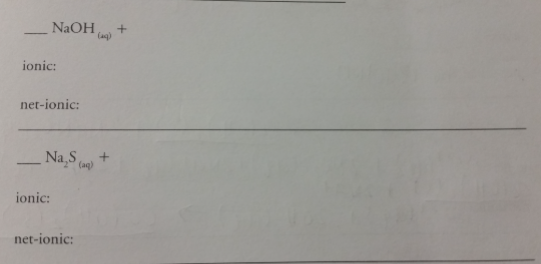
The ionic copper displaces the silver from the silver nitrate, producing an aqueous copper nitrate solution.
The copper silver nitrate reaction. Ammonium sulfate and barium nitrate. In the net ionic equation, any ions that do not participate in the reaction (called spectator ions) are excluded. Single replacement reactions happen in the industry of metal plating and on landmarks, like the statue of liberty.
Posting Komentar untuk "41+ Copper And Silver Nitrate Net Ionic Equation US"